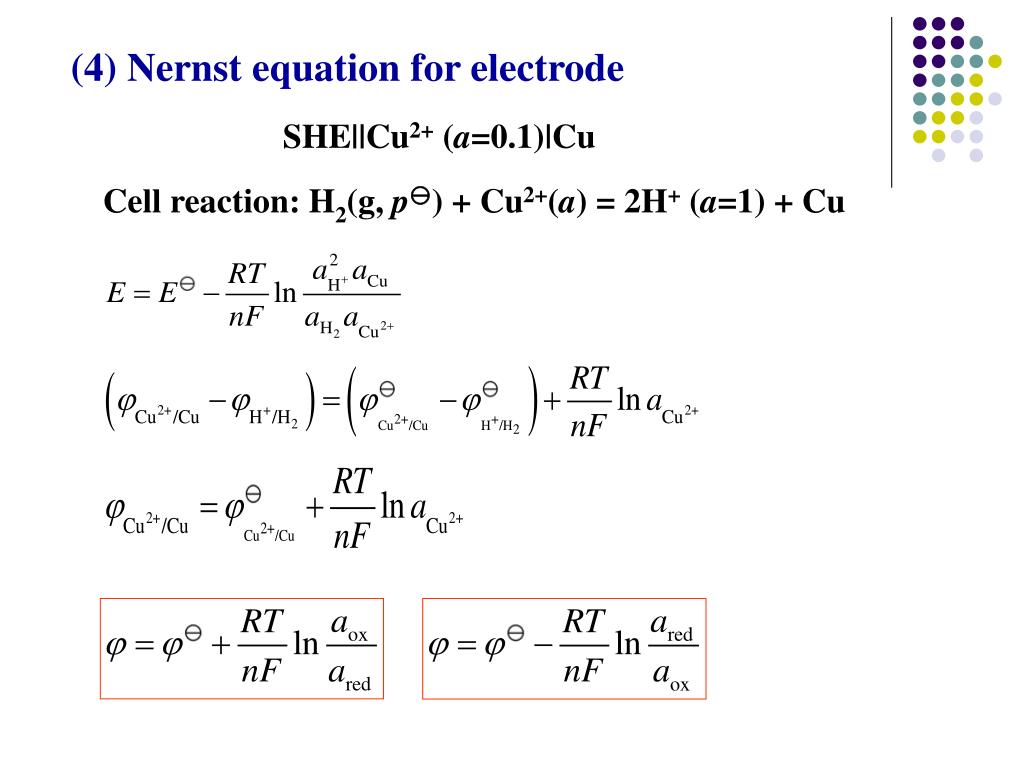
Standard Hydrogen Electrode Nernst Equation . Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (.
from www.slideserve.com
Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0.
PPT Chapter 7 Electrochemistry PowerPoint Presentation, free download
Standard Hydrogen Electrode Nernst Equation Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (.
From www.slideserve.com
PPT Chapter 7 Electrochemistry PowerPoint Presentation, free download Standard Hydrogen Electrode Nernst Equation Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web the nernst. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Electrode Potential ll Nernst equation ll Standard hydrogen electrode Standard Hydrogen Electrode Nernst Equation Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the. Standard Hydrogen Electrode Nernst Equation.
From askfilo.com
3.4. Calculate the potential of hydrogen electrode in contact with a solu.. Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web graphical. Standard Hydrogen Electrode Nernst Equation.
From greenenergymaterial.com
Nernst Equation Electrochemistry Electrolysis Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero. Standard Hydrogen Electrode Nernst Equation.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Nernst Equation Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a. Standard Hydrogen Electrode Nernst Equation.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero. Standard Hydrogen Electrode Nernst Equation.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Standard Hydrogen Electrode Nernst Equation Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web. Standard Hydrogen Electrode Nernst Equation.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Standard Hydrogen Electrode Nernst Equation Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web graphical representation of. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Standard hydrogen electrode ( SHE ) , EC CELL, NERNST EQUATION BY Standard Hydrogen Electrode Nernst Equation Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web the nernst equation. Standard Hydrogen Electrode Nernst Equation.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web suppose a galvanic cell is constructed with a standard zn/zn 2. Standard Hydrogen Electrode Nernst Equation.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Standard Hydrogen Electrode Nernst Equation Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web graphical representation of. Standard Hydrogen Electrode Nernst Equation.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Standard Hydrogen Electrode Nernst Equation Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the nernst equation. Standard Hydrogen Electrode Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6600057 Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Nernst EquationMeasurement of Electrode Potential LN 8 CLASS XII Standard Hydrogen Electrode Nernst Equation Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web suppose. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
CLASS 12 JEE & NEET ELECTROCHEMISTRY NERNST EQUATION & ELECTRODE Standard Hydrogen Electrode Nernst Equation Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web the nernst. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Electrochemistry 9 Nernst Equation Find Electrode Potential or EMF Standard Hydrogen Electrode Nernst Equation Web graphical representation of the onset potentials of the her/hor and oer/orr measured. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web suppose a galvanic cell is constructed with a standard zn/zn 2 + couple in one compartment and a modified hydrogen. Web the nernst. Standard Hydrogen Electrode Nernst Equation.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a Standard Hydrogen Electrode Nernst Equation Web the nernst equation (cell potential equation) relates the reduction potential to the standard electrode. Web hydrogen’s standard electrode potential (e°) is taken to be zero volts at all temperatures, that is, e° = 0. Web in electrochemistry, the nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (. Web graphical. Standard Hydrogen Electrode Nernst Equation.